Excerpted from "Carburetor Fundamentals" by Chevrolet.
WHAT IS A CARBURETOR?
A carburetor is a metering device which mixes fuel with air in the correct proportion and delivers them to the engine cylinders as a combustible mixture. The design of a carburetor is based on the application of natural principles to the job of providing compatible air-fuel mixtures to meet exhaust emission standards and driveability for the varying engine requirements. Just as it is necessary to understand the range of fuel mixtures required for each operational phase, so must the serviceman basically understand the natural forces applied by the design for delivery of these mixtures.
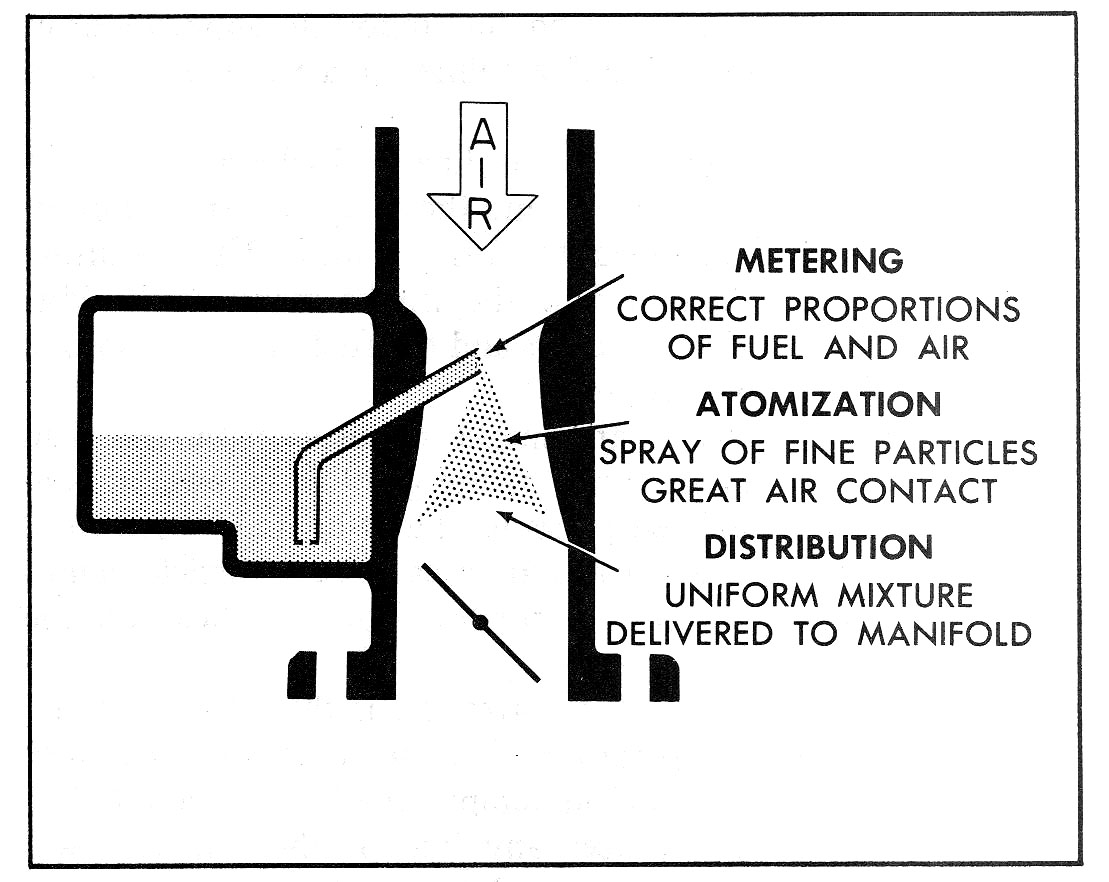
Fig. 1—Basic Functions
PURPOSE OF A CARBURETOR
The purpose of a carburetor on a gasoline engine is to meter, atomize, and distribute the fuel throughout the air flowing into the engine (Fig. 1). These functions are designed into the carburetor and are carried out by the carburetor automatically over a wide range of engine operating conditions, such as varying engine speeds, load, and operating temperature. The carburetor also regulates the amount of air-fuel mixture which flows to the engine. It is this mixture flow regulation which gives the driver control of the engine speed. Regardless of engine speed or load, the carburetor must automatically perform its three basic functions. The automotive carburetor is an intricate device; however, when studied one phase at a time, the functions of the carburetor are easily understood. As mentioned above, the three main functions of the carburetor are to meter, atomize and distribute the fuel.
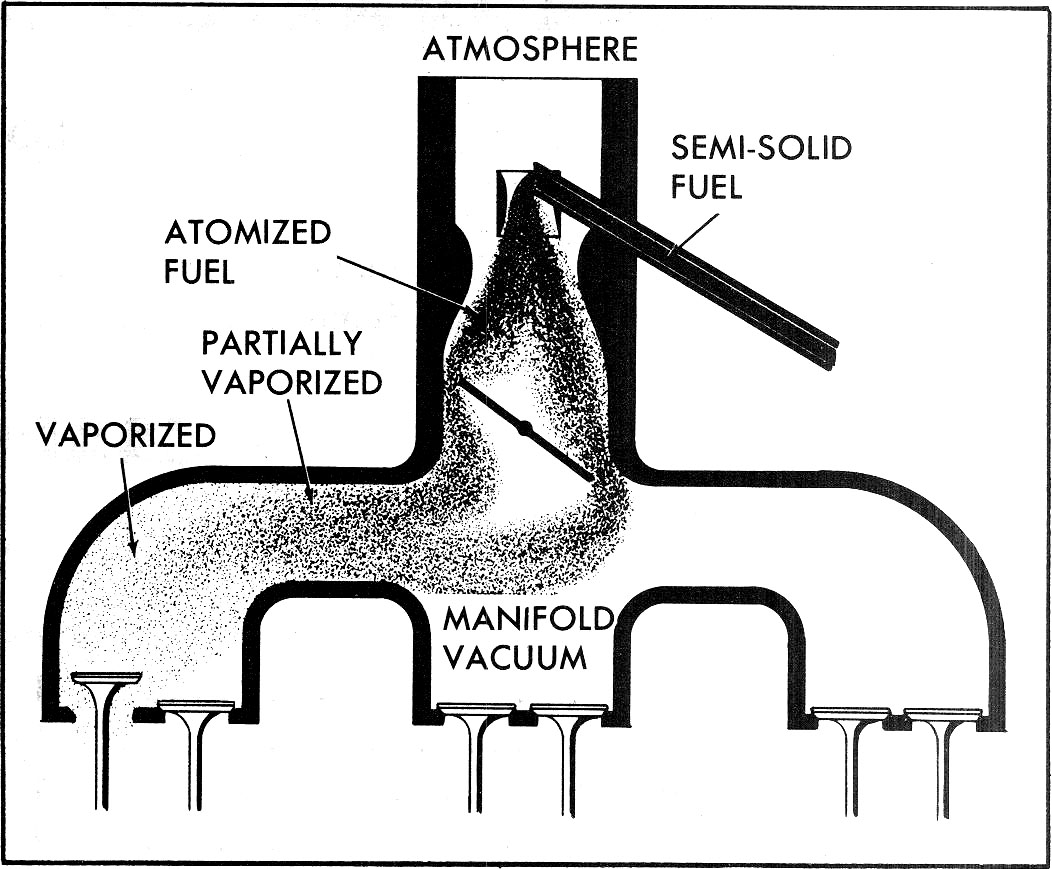
Fig. 2—Fuel Transition -- Atomization and Vaporization Atomization
The engine's source of fuel for power is gasoline. Before gasoline can be used as fuel for an engine, it must be atomized which means breaking the fuel into fine particles so that it can be mixed with air to form a combustible mixture. Contrary to popular belief, gasoline in its liquid state is not combustible; only gasoline vapor will burn. A common example of this is a cigarette lighter which works fine indoors but then can't be lit after you've been outdoors in the cold for awhile. At warmer temperatures, fuels vaporize quickly and so can be ignited easily but at lower temperatures, evaporation is slower and accordingly ignition is impossible or, at least, difficult because of insufficient vapor. While this analogy is quite simple, it is an illustration of the basic problems of carburetor design; that is, the provision of combustible fuel mixtures over a broad range of temperature and operating conditions.
The complexity of design problems continues to mount each year due to a constant effort to reduce exhaust emissions and at the same time improve operating conditions. To be combustible, gasoline must vaporize. Vaporization is the act of changing from a liquid to a gas and this change of state occurs only when the liquid absorbs enough heat to boil. This is what happens in a tea kettle to change water to water vapor, or steam. Heat is transferred to the water, raising its temperature until it finally reaches the boiling point, at which time the water changes to steam and is carried off to atmosphere in this gaseous form.
At sea level, the water will boil at 212°F but at high altitudes, less heat is required for water to boil due to lower atmospheric pressure. This is known as the temperature-pressure relationship; that is, as the pressure is reduced, the boiling point is reduced. This law, combined with a process known as atomization, has important applications in the transformation of liquid gasoline to a vapor for use in combustion. In a carburetor (Fig. 2), gasoline is discharged into the incoming air stream as a spray and the spray is then atomized, or torn into fine droplets to form a mist. The resulting air-fuel mixture is drawn into the intake manifold. At this point, the change of state occurs and the fuel "mist" vaporizes as the result of several factors.
Since the pressure in the intake manifold is far less than atmosphere, the boiling point of the gasoline is lowered considerably. At this reduced pressure, latent heat absorbed from the many air particles surrounding each fuel particle causes some vaporization, which is further aided by heat on the intake manifold floor. Because complete fuel vaporization is the result of many factors (ambient temperature, fuel temperature, manifold vacuum, and intake manifold temperature), it is easy to see that anything which reduces any one of these factors will adversely effect vaporization and thus reduce fuel economy and increase exhaust emissions. Some examples would be cold weather, an inoperative exhaust heat control valve, and high overlap camshafts and/or heavy throttle demands.
While the effects from lower temperatures are obvious, reduction of manifold vacuum either by valve timing or heavy throttle operation are highly detrimental to fuel economy due to the higher pressures (and boiling points) resulting in the intake manifold which reduces the amount of fuel vaporization which will occur by the time the charge enters the combustion chamber. Fuel not vaporized at the time of induction is, to a large extent, exhausted unburned from the combustion chamber and can cause high hydrocarbon exhaust emissions.
We know that gasoline, for combustion, must be vaporized or as the change of state can be loosely considered, absorbed by air. However, this requirement is further limited by the amount of air that the fuel vapor is absorbed by. Combustible mixtures in an engine are limited by the following proportions, or ratios, of air to gasoline: eight parts air to one part gasoline is the richest mixture that will fire regularly and a mixture of 181/2 parts air to one part gasoline is the leanest mixture that will fire without missing in an engine. Mixtures leaner than 18.5:1 tend to cause misfire. From an automotive standpoint these ratios represent the mixture extremes that an engine can tolerate, but these limits do not provide the two conditions most sought. The most desired ratios are a mixture that will produce the most power per pound of gasoline and a mixture that will provide the best economy or most miles per pound of fuel with the least exhaust emissions.
next: Fuel Metering
